Introduction
Metabolomics is an emerging tool to study metabolic alterations in cancers. It has also the potential to identify clinically relevant biomarkers for diagnosis, risk appraisal, and targeted drug development. In hematology, metabolic reprogramming is essential for normal regulation of hematopoiesis. Association of discrete metabolic alterations with explicit somatic mutations have also been reported in hematological malignancies. We have previously observed glutaminase (GLS) overexpression and improved oxygen consumption rate with excess glutamine in low glucose conditions in patient-derived fibroblasts in ERCC6L2 disease (unpublished). This prompted us to study metabolomic alterations in ERCC6L2 disease and Shwachman-Diamond syndrome (SDS) induced strictly by the germline ERCC6L2 and SBDS mutations. Both inherited bone marrow failure (BMF) syndromes carry considerable tendency of somatic TP53 mutagenesis predisposing patients to a high risk myeloid malignancy. Our aim here was to identify potential drug targets by targeted metabolomics.
Methods
We examined patient-derived skin fibroblasts (ERCC6L2 disease, n=4; SDS, n=4) and healthy controls (n=4) and performed targeted metabolomics with U-13C-D-glucose and U-13C-L-glutamine labeling. Fibroblasts were seeded on six-well plates and on the following day exposed to 13C-glucose (25mM) or 13C-glutamine (2mM) label containing medium for 30 or 120 minutes. Next, metabolites were extracted and quantified with liquid chromatography mass spectrometer. The results were normalized to total metabolite intensity per sample.
Results
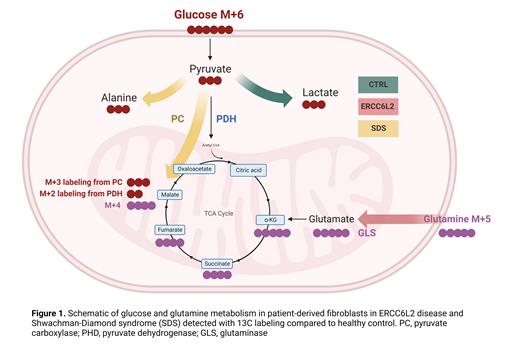
In 13C-glucose labeled samples, we detected statistically significantly lower intensity of pyruvate M+3 in both ERCC6L2 and SDS fibroblasts compared to control at 30 minutes, which persisted at 120 minutes in SDS cells. In the TCA cycle, malate M+3 labeling indicates pyruvate carboxylase (PC) activity and M+2 labeling pyruvate dehydrogenase activity (PDH) (Figure 1). We found a significantly higher portion of malate M+3 than M+2 in SDS cells at all time points, suggesting lower PDH and higher PC activity in SDS cells. Additionally, we detected higher levels of alanine M+3 in SDS samples. As pyruvate is a source for alanine synthesis, we suspect an alteration in pyruvate metabolism; potentially to overcome challenges in pyruvate oxidation due to either decrease in the respiratory chain function or PDH activity.
In 13C-glutamine labeled samples, we detected statistically significantly higher intensity of glutamine M+5 and M+4 in ERCC6L2 samples at 30 minutes compared to control and SDS cells. Glutamine is first converted to glutamate by glutaminase (GLS), after which it enters the TCA cycle as ketoglutarate (KG) (Figure 1). In addition to glutamine, we detected an increase, although not statistically significant, in glutamate M+5 and M+4, as well as KG M+5, in ERCC6L2 cells at 30 minutes. A similar trend was observed in the subsequent TCA cycle intermediates succinic acid M+4, fumarate M+4, and malate M+4. At 120 minutes, there was no difference in metabolite intensities between the groups, suggesting a potentially faster glutamine uptake by ERCC6L2 cells.
Conclusions
Our analysis using non-hematopoietic tissue provides understanding of metabolic weak links not confounded by somatic events occurring early in the disease course in ERCC6L2 and SDS patients. Our findings imply increased glutamine utilization in ERCC6L2 cells and altered pyruvate metabolism in SDS cells. Therefore, these metabolic pathways are attractive drug targets (summarized in Figure 1). Further studies are needed to determine their potential in supporting the compromised hematopoiesis to reduce the stress for somatic mutagenesis.
Disclosures
Wartiovaara-Kautto:Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Genzyme: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal